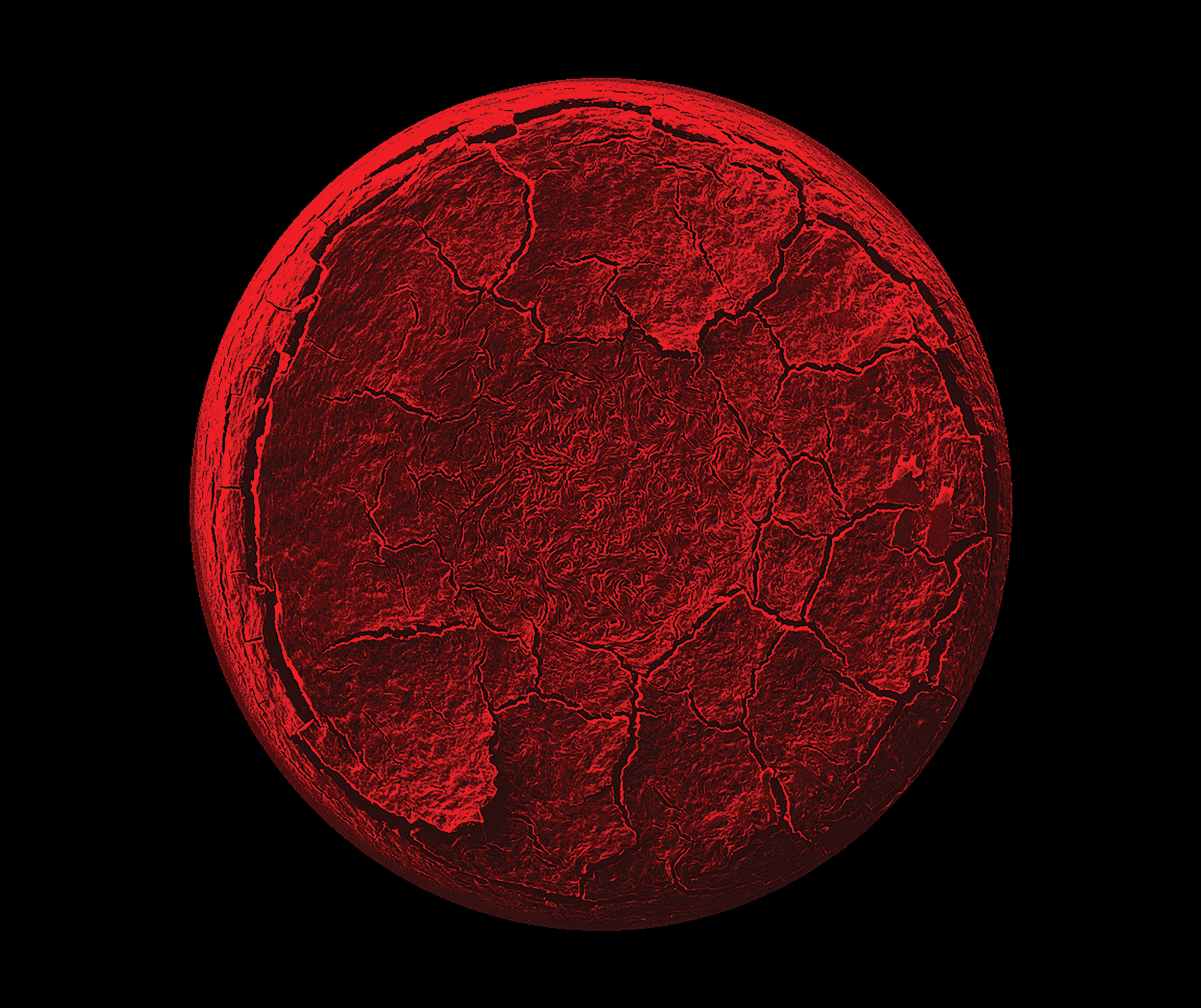
Penn researchers, led by Arjun Yodh, James M. Skinner Professor of Science in the Department of Physics and Astronomy and Director of the Laboratory for Research on the Structure of Matter, recently identified complex behavior in a drying liquid crystal drop. This drying process is different from that of a drying coffee drop, another liquid the researchers have studied. The differences arise because of the character of liquid crystals, fluids with aligned phases of constituent molecules. The formation of different phases during drying, each with unique structure and symmetry, leads to novel fluid movement and solid deposition.
The study also provides insight useful for control of drying solutions of macromolecules that occur in dyes and pharmaceutical formulations. This photo is an electron microscope image (colorized) of a dried liquid crystal drop whose final shape resembles a volcano or sunken soufflé. The shape is due to convective flows that carry material towards the drop edge. The central thinned region locks in the turbulent flows present in the drop just before final formation of the more viscous liquid crystal phase.