Ever wondered what exactly is going on inside a cooking egg to change it from its clear goopy consistency to an edible white? Like the majority of cellular activity, a protein is front and center. In this case, however, the protein is actually behaving erroneously, misfolding, in order to go through its metamorphosis.
"DNA is the permanent record for everything in our body. Proteins, made up of amino acids, are doing the real work," says James Petersson, Assistant Professor of Chemistry. "You can think of these amino acids as beads on a string. These beads have properties that cause them to interact in certain ways, and therefore fold into specific shapes. It is the protein's shape which ultimately defines its function."
While pursuing his doctorate, Petersson studied the proteins that are responsible for the communication between neurons. After earning his Ph.D., he continued along this research path with synthetic amino acids, testing whether proteins would still hold up if their natural backbone had been almost completely replaced by man-made materials.
"Following my doctoral and post-doctoral research, the Penn Chemistry department was a natural choice given its renown in organic synthesis. It was also important to me to have a research hospital nearby that was very involved in biophysics and other potential applications of the work we do."
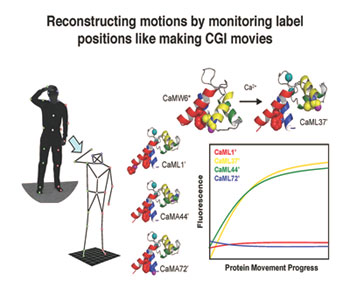
When Petersson came to Penn he decided to head in a new direction with the exploration of not just synthesized protein building blocks, but entire synthesized proteins. Petersson's lab has developed a unique way of tracking protein movement, one that involves a subtle change to an amino acid. All amino acids are connected by an amide bond. Petersson's lab takes this bond and substitutes sulfur in for oxygen—a single atom substitution that is a very subtle change to the protein overall. These modified amide bonds, called thioamides, don't emit light, but they can quench fluorescence from other probes and thus be used to chart the movement of the protein. The key, Petersson says, is to cram as many probes into as many locations as possible.
"In motion capture for CGI movies, you have a guy in a suit with a bunch of labels and the camera tracks those and tries to reconstruct movement. This is essentially what we want to do with proteins: to be able to track the labels and reconstruct the motions. Say you have a small protein with multiple helices, if you place probes in certain ones and track the distance between them using thioamides, it reveals how they're moving and communicating. And just like with motion capture, the more labels you have, the better the tracking."
"In motion capture for CGI movies, you have a guy in a suit with a bunch of labels and the camera tracks those and tries to reconstruct movement. This is essentially what we want to do with proteins: to be able to track the labels and reconstruct the motions. " – James Petersson
Protein tracking has very real implications, one of which involves the synthesizing of misfolding proteins in diseases such as Alzheimer's. Amyliod-β, for instance, is normally a monomer, but with Alzheimer's it starts to cluster, creating fibers that stack. The fibers then form long strands, choking neurons off. By introducing the thioamide and fluorophore into a synthesized Alzheimer's protein, Petersson's lab is tracking the toxic misfolding of these proteins.
"We see similar misfolding in prion diseases like mad cow. Once one misfold occurs, it propagates other misfolds—a domino effect if you will. Using our methods, it's possible we can recreate models of these diseases as well, in hopes of gaining insight into how the proteins behave."
Petersson says the future is wide open when it comes to the use of protein tracking techniques. Eventually labs like his may be able to use similar fluorescence-finding methods to set off reactions in antibody samples that could help diagnose diseases like HIV/AIDS.
"The mystery surrounding the folding of amino acids into specific proteins is a hugely important question in biochemistry, one our lab, along with many others around the world, are working everyday to solve."